This is what we found in our jar when we came back to the lab today:
Monday, December 14, 2015
Lab Day 3 Calculations
Ok. Today was the last day to finish up our Copper lab which, I thought was one of the more fun labs we've done so far this year. We made sure to measure the mass of both the baby food jar and the nail again so that we could subtract those masses from our previous calculations. From that we needed to calculate these things: data table, theoretical yield, actual yield, percent yield, and be able to determine which iron ion was formed.
Sunday, December 13, 2015
Preparing for the Unit Test
In order to prepare for this unit test, I made sure to again reference the infamous Crash Course Chemistry YouTube channel. After doing that, I completed the calculations for the 3-day lab we had finished today which went over again the concepts we need to know for the test. Since my teacher moved the test back a day, I felt like I was able to find and use more tools to prepare. Because it wasn't on a Monday, I had a chance to get in the swing of the class again which is nice to kind of wade in instead of just making a big splash. This time, I made sure to pick up my quiz from last week and looked it over to see what I had made mistakes on and what I could improve upon for the unit test. Again I utilized the all-powerful schoology to use the practice tests and check my understanding of the unit one last time.
Thursday, December 10, 2015
Copper (II) Chloride and Iron Lab
We're on a streak! Hamming and I passed this pre-lab quiz yet again, so we got to participate in what I would say was a pretty fun lab. We first had to measure out Copper (II) chloride into a weigh boat and pour it into the baby food jar and added water. Then, we weighed the nail and then set it into the solution and left it overnight. This is what it looked like at the end of the hour on that 1st day:
The second day, we made sure to siphon out the excess water and made sure to leave as much of the product in the jar as we could. Then we added 25mL of HCl and siphoned that off after a while as well, again making sure to leave the product. This is what the jar looked like when we got into class on the 2nd day of lab:
Helpful Links
As we started out this unit, I found these few links to help me get acquainted with the concept. Hope these help for you as well!
Worked out example
Khan Academy Example
Chem Collective Practice
Worked out example
Khan Academy Example
Chem Collective Practice
Thursday, December 3, 2015
Helpful Videos
Here are some other beneficial videos for this unit that might help:
Crash Course Redox
Bozeman Redox
Crash Course Acid Base Reactions
Crash Course Precipitation Reactions
AP Chemistry Acid Base Reactions
Crash Course Redox
Bozeman Redox
Crash Course Acid Base Reactions
Crash Course Precipitation Reactions
AP Chemistry Acid Base Reactions
Wednesday, December 2, 2015
Overview of Lectures
Whew. Thank goodness we have another unit test over and done with! The exam wasn't horribly difficult, and I think I improved my score from my last unit test and the last quiz we took. Just to recap the avalanche of information we learned over the past few weeks, I had gotten a review sheet from a friend to help study off of, which was extremely helpful in getting all of the information condensed into one piece of paper. This unit we had covered redox reactions (synthesis, decomposition, single-replacement, and combustion), balancing chemical reactions, predicting products of a chemical reactions, oxidation numbers, reactivity series, net and complete ionic equations of reactions, what signals a chemical reaction, and acid base reactions. Wow, that's actually a lot more topics than I had expected, but after learning them, hopefully I will recall them all easily for the final coming up very quickly.
Preparation for the Unit Test... Again
In order to improve my unit test score, I printed out some practice tests from schoology and completed them with a friend constantly comparing answers and making sure that each of us knew exactly what was going on in each reaction. I feel pretty prepared for the test, more so than the last unit test. A friend also helped me put together a review sheet that highlighted the main points of the unit and listed the polyatomic ions, which now, I feel like a have a solid grasp on finally. I feel that the labs which covered the balancing and predicting of chemical reactions played a big part in the continuous review process. As the year has been progressing, I'm getting more and more used to a test or quiz each week, and even though they are a total pain, they are effective in letting students know exactly what they need to focus on to be better the next assessment.
I also made use of the always reliable Crash Course Chemistry videos especially this one below discussing redox reactions:
Metal Lab Excitement
Yesterday during class, after our pre-lab quiz, Hamming and I set off to lab again to set off some pretty cool reactions. We were able to use the well plates again and set elements into them and use a pipette to put the solutions in the well plate. This lab was extremely similar to the one we had completed previously, but focused more on reactivity series, but still reviewed our knowledge of net ionic equations after completing the lab. Although we passed the pre-lab quiz, those who didn't also got to get into the lab, which, I thought, was great because even though they got the question wrong, they were aware of what they made a mistake on after an explanation, and they also got to participate in an awesome lab.
Reactivity series:
Monday, November 30, 2015
Lab of Epic Proportions
We did a lab a little while ago, but I forgot to post about it so here we go. After passing the pre-lab quiz, which wasn't too complicated this time, but involved predicting the products in a reaction, Hamming and I set off to the lab station. We were to combine 2 reactants in a well plate and watch the reaction occur and record which ones formed a product and which ones didn't. Many of them didn't have reactions, but some of the reactions were pretty cool. In order to complete the rest of the lab, we had to complete the net ionic equations that reflected the outcome of each reaction that DID occur. Doing this was good practice for doing this type of problem, which will definitely be on our unit test Thursday.
Friday, November 13, 2015
Preparing for the Unit Test Monday
In preparation for our test on Monday, I really need to review the information more than usual. I also need to complete the rest of the suggested homework because my quiz score reflected my lack of core knowledge on this unit despite my studying. I think I will get together with some friends in a sort of study session fashion and bounce ideas off of each other so we can all have a better understanding of this unit. I tend to find that other peers know how to put the information into terms and ways that I am able to easily understand. I will also make sure to look over my notes from previous lectures in this unit and from other units to help me remember those concepts for the cumulative review. Overall, the quiz we took this week was a wake-up call for me to realize that I need to know the unit inside and out in order to achieve better scores on the test and remember the information for the final.
Thursday, November 12, 2015
Formula of a Chloride Lab
Today was a good day. I got into the lab, and it turned out to be a fun experience. But, since Hamming wasn't there, it was a shame I didn't get a chance to work on it with him. Mackenzie and I, though, were able to complete the lab with an extremely small percentage of error. We took the correct measurements and used them with the skills from yesterday's lecture in order to calculate the empirical formula of the zinc chloride compound. Overall, these calculations were much more fun to make since we actually gathered the data for ourselves this time.
Below, you can find some pictures from the lab today:
Monday, November 9, 2015
Lab Disappointment With a Hint of Reassurance
Today, my lab partner and I attempted to complete the pre-lab question in order to get into the "Formula of a Hydrate Lab". My part of the question was fairly simple since it was just explaining what LD50 meant, which is 50% of the lethal dose of the given substance. Hamming's part was much more complicated as he had to complete the conversions for the lab and identify the chemical formula used in the lab. We ended up not passing the pre-lab and were not able to complete the lab, and we were fed data. Although sad, we were able to practice the conversions and I was very pleased that I knew what I was doing with that and got the correct answer. This whole thing was very good practice for our quiz tomorrow that will prove to be challenging because we will have a lot of conversions to complete in not a substantial amount of time. I will need to make sure to review polyatomics and naming in order to feel confident going into the quiz tomorrow!
Below you will see a quick review of these polyatomic ions we need to know:
Monday, November 2, 2015
Chemical Composition Pre-Test Woes
Today in class, we took our Pre-Test for the Chemical Composition Unit. Some of the things on the test were familiar because we had briefly touched on mols with "Mole Day" being 2 weeks ago and our project we were supposed to do for that. Because of this, I'm sure I got at least 1 question right because it referred to Avagadro and his ownership of the unit of moles and I remembered that from looking up funny mole puns to use for my stuffed mole project. Overall though, I feel that I have a lot to learn in order to succeed in this unit and I'm ready to feel confident on these upcoming quizzes and tests!
Thursday, October 29, 2015
Mole Day 2015
On Friday, October 23, we celebrated "Mole Day" in class. I made a "Cave-mole" and I actually thought it turned out pretty well. Everyone else had clever and very cute ideas about what to make their mole as. We were given a pattern beforehand which made it a lot easier to figure out and I used a sewing machine, unlike other people, which took a lot less time. Seeing all the moles on the back table at the end of the day was amazing because our ideas and interpretations were so different and that was really interesting to me.
Below you can see all the fabulous moles everyone made from most classes:
Wednesday, October 28, 2015
Changes Activity
Last Wednesday, we went into the lab stations and completed a fun, yet challenging activity to reinforce what we had learned in the lecture just a few minutes before. We were told to go to each of the eight stations and solve each substance with the already given elements, solutions, and mixtures in their specified column. I really enjoyed science in middle school and we had worked with chemical and physical changes frequently, so this came as sort of second nature to me. It was also a great idea to immediately review the topic covered, especially in a creative way, using common objects and using the help of a partner of our choosing.
Below you can find a few of the stations we had to go through and attempt to solve:
Below you can find a few of the stations we had to go through and attempt to solve:
Tuesday, October 27, 2015
Dimensional Analysis Lecture
Today, we went over the dimensional analysis portion of the chapter which felt like a review for me because I had done it recently during Trigonometry. After that, we reviewed density briefly and also learned the conversion for Kelvins. I found that to be the most confusing because I had never used Kelvins before because I had only ever used conversions for Farenheit and Celcius. The test is Thursday which is very soon, so today after I go home I will definitely reviewing all our information so far and maybe complete a few practice sheets and quizzes.
Monday, October 5, 2015
Aspirin Lab Anxiety
Oh my goodness, I am extremely nervous for Cristen and my turn to complete the lab on Wednesday. Only one group in our hour successfully completed the pre-lab question, meaning only one group out of the five were able to actually complete the lab at all. Because of this, I will make sure to study the lab instructions very carefully and know the entire procedure inside and out. If I am able to do the lab, I feel that it will be a fun and interesting learning experience to deal with harsh chemicals and to be a part of making real aspirin. But, if I do not pass the pre-lab, then this experience will be taken away and I will just be given raw data with no explanation.
Here you can see a very helpful video of what I will preparing for in the next few days.
Here you can see a very helpful video of what I will preparing for in the next few days.
Thursday, October 1, 2015
Post-Unit Reflection
Today we took our Unit assessment over chapters 3 and 11. I felt pretty good about most of the questions and the test didn't take me too long to complete. Since it is the end of the unit, we now need to have completed our guided readings, blog post requirements, and our most recent project about elements in the stars. It was definitely a lot to accomplish in a short amount of time, but it was all a good way to teach us to manage our time and keep up on daily happenings. Tomorrow we will be previewing the aspirin lab, which we were supposed to do a few weeks ago, so I am very anxious to see what we will be preparing to do on Monday!
Wednesday, September 30, 2015
Forensic Archaeology Lab (Graphing and Post Lab)
Yesterday, we completed the graphing aspect of the lab. My computer was not loading fast enough, so I got behind very quickly and was scrambling at the end to complete the graph. The actual graphing was simple, but the process of editing the graph was very technical and required a lot of different steps. Then, we were told to use our graph to complete the rest of the lab to find the missing person. Cristen and I concluded that it must be Sue Crayton because her body and time of death fit most with the amount of decay. I thought it was pretty cool that we combined forensics with chemistry even though someone had to die in order for us to do this...:). We were told them to complete the rest of the post-lab questions, so I will finish those tonight and then submit them to SCHOOLOGY.
Here you will find the post lab questions and data table we had to complete:
Monday, September 28, 2015
Forensic Archaeology Lab (Data Collection)
For this lab, we were told to gather 1 sheet of 12x12 paper, 2 pairs of scissors, 1 ruler, and 1 plastic cup. After getting the materials, I was very curious to see what we were actually going to be doing with all of these things. We were to draw a 24x24 grid on the white side of the paper using the ruler and after, should cut them all out individually. This proved to be a very tedious task. When done cutting, we threw away 9 squares immediately in order to have the necessary 567 total squares. We put the squares into the plastic cup and shook it up, then poured them out on the desk. Then, we separated the squares into two separate piles- white side up and orange side up. After the separation, we counted the number of atoms that decayed (orange side up), recorded that data, then subtracted that number from the starting number of squares (567). For the next decay, we threw away those that landed orange side up and put the white side up ones back into the cup to repeat the process. We repeated this 6 times total. Even though most of the work was tedious, I really enjoyed this example of decay because I was able to visualize the half life process. Tomorrow we are going to be graphing the data collected, which is exciting because, for some reason, I really like to GRAPH, and here's a link to a brief preview for tomorrow.
Here you can see the pieces of paper used to complete the lab and the data collected.
Wednesday, September 23, 2015
Post-Quiz Reflection
After about a week of Atomic Structure and Radioactivity, we took our first quiz over the material. I studied all of the material last night and reviewed my polyatomics :), to review to make sure I was ready to take the quiz. I felt confident about the review material, which felt really good. The new material was fairly easy as well; the scientists' models clicked and I breezed through the matching section. I'm actually coming to like this section and I'm ready to learn more. Overall, I think I'll succeed in each of the objectives and hopefully this will help me for other quizzes and tests to come.
Here you will find the Learning Objectives highlighted in this unit:
Tuesday, September 22, 2015
Beanium Lab
Today, we were told to complete a lab in order to calculate the average mass of the isotope, the percent abundance, and the total mass of all the atoms of the isotope, which was appropriately named Beanium. This new element is shaped like a bean and can actually seen by the naked eye. During the lab, we weighed each of the different isotopes (colors) of beans, making sure to tare the scale, and used that to find the total mass of this isotope. Then, based on the number of atoms of the isotope present we were able to divide that number by the total number of beanium atoms in the sample. I really liked this lab because it was a fun and simple way to further review the concept of calculating average atomic mass and it had a clever lead in to explain the nature of the lab.
(Here is a basic example of how to calculate average atomic mass)
(Here you can see the different Beanium isotopes and the cup we put them in to measure their mass)
(And finally, HERE you can refer to this example of the whole Beanium lab expalined in great detail.)
Monday, September 21, 2015
Find Someone Who...
Today in class, we had our second lecture day. It was rough. I forgot my calculator, so that was my first mistake. We learned how to calculate the number of protons, neutrons, and electrons in an element and its isotopes as well. At the end of the lecture, we broke off and completed a worksheet where we had to find someone who knew the answers to the questions asked on the sheet. All of the topics were review, but I realized I hadn't retained much information from the first lecture, but I readily knew the information we learned today. It was a wake-up call to start reviewing for the upcoming quiz on Wednesday, which will contain some items for review. I think doing the cumulative review is a great idea because that way, we are constantly reviewing and retaining the information that we need to know for the final. In this link HERE, you can see the documented benefits of using cumulative review.
Thursday, September 17, 2015
Obscurtainer Activity
Using the activity during class yesterday, I was able to mimic RUTHERFORD'S model of making inferences to determine his atomic model. We were told to make an educated guess of the shape inside the closed cylinder that the ball was hitting off of, which proved to be very difficult. After a few tries I got more accustomed to the basic shapes inside and I actually guessed a few correctly. Through this, I was experiencing the guessing process used by this revered chemist for his view of the atomic model.
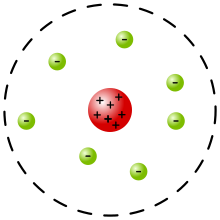
Rutherford's Model
Wednesday, September 16, 2015
Lecture/Notes Day 1 Reflection
After our first day of atomic structure and radioactivity notes, I have a basic understanding of the changing atomic theory. From Dalton's atomic theory, which came to have 1 1/2 postulates refuted, to Thomson and Rutherford's which brought up the discovery of subatomic particles through two ground-breaking experiments. These all led to the present day model of the 3D atom with the nucleus containing protons and neutrons and negatively charged electrons surrounding it. With this knowledge in mind, I think it will be easier to process other information regarding atomic structure in the days to come. A brief overview of the history of the atom can be found HERE, but contains an earlier history and a later history than those discussed in the lecture in class.
Monday, September 14, 2015
Real World Uses of Binary Compounds and Acids
Knowing the uses of binary compounds and acids proves to come in handy in the real world for many reasons. For example, in the grocery store assignment we completed, we were told to name various compounds and acids based on its description and properties, then find common items containing them. This project let me realize that Chemistry can be applied to everyday situations even though we might not know it. And to think, students always claim we won't use the information we learn in school, in real life! Distinguishing compounds and acids in foods or other products could be vital in maintaining someone's health and could possibly used to save their life.
Frontier Chemistry Nomenclature Reflection
I found over the course of the Frontier Project, that I had no previous knowledge about local flora in the least. Through this project, I was able to effectively distinguish between plants and quickly treat a list of maladies. I wasn't able to say that before! The most difficult part of the task was making sure that I had all of the requirements in my blog and was properly equipped for the constructed response essay in class. Although that was difficult, I really enjoyed problem solving in a real life situation where I used the information collected outside of class to show my growth through the whole process.
Thursday, August 20, 2015
Introduction Page
Hello! I am a high school student currently enrolled in Pre-AP Chemistry. I plan to go on to a 4-year university after high school and study either Psychology or History and acquire a sustainable career in whatever field I choose. I enjoy reading, writing, traveling, and learning. My interests include music and history.
Follow me on Twitter : https://twitter.com/Gigisteeno
or Instagram: https://instagram.com/gillianmsteeno/
Subscribe to:
Comments (Atom)